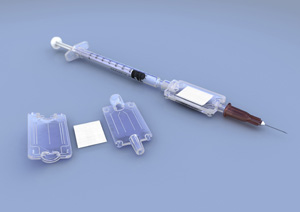
Image courtesy Nova Bio-Pharma Technologies
Vaccines have dramatically impacted global health: by 1979, international vaccination campaigns had successfully led to the eradication of smallpox, which was once estimated to kill as many as 30% of people infected. And since the launch of the World Health Organization’s polio eradication campaign in 1988, widespread vaccination has contributed to a 99% worldwide reduction in the disease, which now persists in just four countries: Afghanistan, India, Nigeria and Pakistan. Yet, the potential for live vaccines—which contain a version of a disease microbe that has been altered in a lab to promote the production of antibodies, but not cause actual infection—to effect even greater change, and combat diseases particularly in the developing world, has long been limited by the fact that preserving these types vaccines means keeping them refrigerated. (Inactivated vaccines, in contrast, can often be freeze-dried and don’t need to be refrigerated, but they generally produce a weaker immune response and require more follow-up shots.) In regions without widespread electricity grids, especially in tropical climates, this has been particularly problematic. Yet, new technology developed by British firm Nova Bio-Pharma Technologies and tested at Oxford University, may represent a sea change in vaccine preservation—minimizing the need for long-term storage in refrigerators or freezers, and improving access and global health as a result.
In findings published this week in the journal Science Translational Medicine, the technology, which utilizes special membranes stabilized with a sugar compound, was shown to preserve two different types of virus-based vaccines for four months at a temperature of 113ºF (45ºC), with no degradation. At slightly lower temperatures, 99ºF (37ºC), the technology kept vaccines preserved for up to a year, losing tiny amounts of the vaccine to absorption in the membrane. When the vaccine is needed, the membrane is simply attached to a conventional syringe, flushed with a solution, and injected into the patient.
The technology is called HydRIS, which stands for Hypodermic Rehydration Injection System. The promising new technology could mean a world of difference to vaccination efforts—making it easier to transport and store vaccines, without the need for refrigerated trucks and monitored refrigerated medical storage facilities. Enabling warmer temperature storage and transport of vaccines could make a dramtic difference in the developing world, where cold storage facilities and transportation options may be spotty or non-existent in certain places. But, in the developed world too, where those facilities are more readily available, reducing the need for refrigeration could drive down costs—keeping vaccines refrigerated through transport and storage costs some $200 million annually, increasing the price of individual vaccines by 14%, according to WHO estimates.

